Research Overview

Our research combines electrophysiological and morphological techniques in brain slices to focus on understanding the mechanisms underlying neuronal dysfunction in the basal ganglia and cerebral cortex in neurological disorders including Huntington’s disease in animal models and epilepsy in children with malformations of cortical development.
More recently, our research uses advanced methods to visualize live neurons in freely moving animals with the aid of miniscopes and calcium reporters.
Projects
Cortical Pathophysiology in Mouse Models of Huntington's Disease
The fatal mutation in Huntington’s disease (HD) leads to an expanded glutamine repeat within the huntingtin protein which causes neuronal dysfunction typically followed by selective neurodegeneration especially within the striatum and cortex. These dysfunctions in neurons and circuits occur during the development of the disease phenotype, well before there is significant cell loss.
Recent studies in animal models have emphasized that synaptic cell-cell interactions play a role in the pathophysiology of this disease. For example, removing mutant huntingtin from the cerebral cortex ameliorates some HD symptoms. The experiments in this project are designed to understand the functional changes that occur in specific populations of cortical neurons during the progression of the HD phenotype and to uncover new targets and approaches for therapies.
Two-photon calcium imaging in the motor cortex of HD mice while running on a treadmill. The red cells are interneurons that genetically express tdTomato. Green cells are cortical neurons that virally express GCaMP6f. Interneurons that co-express GCaMP6f are seen as yellow cells.
Cortical Maldevelopment Contributes to the Pathophysiology of Huntington's Disease
Although HD typically starts in adulthood, higher CAG repeat numbers produce a shift in disease onset which can start in juveniles and even infants. Juvenile HD is more severe than adult-onset HD and the symptoms, including rigidity, mental retardation, and seizures differ from those typical of adult-onset HD, namely chorea (uncoordinated dance-like movements), cognitive deficits, and mood swings. These symptoms are caused by cell dysfunction and loss primarily in striatum and cerebral cortex. The cortex of the WT mouse showed normal architecture and well delineated layers.
In contrast, the cortex of the R6/2 mouse appeared dyslaminated and some areas were devoid of NeuN immunoreactivity while superficial areas showed neuronal crowding, suggestive of cortical dysplasia.However, at present nothing is known about the early development of cortical and striatal morphological and functional abnormalities in HD. This project will determine how early during brain development aberrant cell membrane properties and synaptic communication can be detected in cortical and striatal projection neurons. Particular emphasis is placed on trying to understand the origin and mechanisms of cortical hyperexcitability in HD, as well as finding methods to restore normal function.
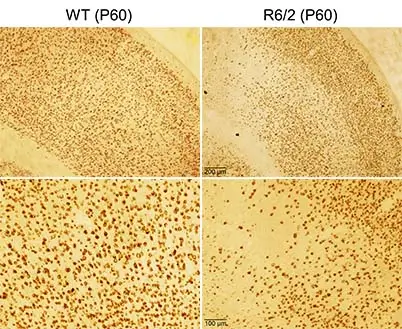
WT and R6/2 mice (P60) were perfused and corticostriatal slices were processed for NeuN immunohistochemistry. The cortex of the WT mouse showed normal architecture and well delineated layers. In contrast, the cortex of the R6/2 mouse appeared dyslaminated and some areas were devoid of NeuN immunoreactivity while superficial areas showed neuronal crowding, suggestive of cortical dysplasia.
Basic Mechanisms of Pediatric Epilepsy
The developing brain is a very delicate and sensitive organ. Even small errors in cell generation, proliferation, and migration can lead to devastating consequences. This delicate balance can be easily perturbed by physical and metabolic insults, explaining the peculiar susceptibility of developing brains to become hyperexcitable and potentially epileptogenic.
A number of important differences between young and adult brains have been recognized for a very long time. This implies that therapies that are useful in adults may not adequately control seizures in young epileptic brains.
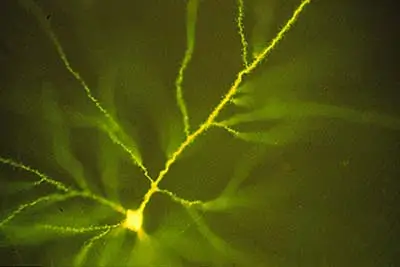
Cortical pyramidal neuron recorded in a tissue sample resected from a child with cortical dysplasia for the treatment of pharmacoresistant epilepsy. The neuron was filled with a fluorescent dye, Lucifer Yellow.
Identification of Striatal Neuron Populations Underlying the Effects of Opioids Using Miniaturized Microscopes and Calcium Sensors
This project takes advantage of two recent technological advances to study specific striatal neuronal populations involved in mediating the effects of opioids. The first of these advances makes possible the manipulation of the activity of specific cell populations by photostimulation.
The second has permitted the creation of miniature microscopes (miniscopes) with which to visualize the neuronal activity in deep regions of the brain, such as the hippocampus or striatum. In this project, we adapted this technology to study specific striatal neuronal populations involved in mediating the effects of opioids.
Calcium transient activity of striatal indirect pathway medium-sized spiny neurons visualized using a combination of viral GCaMP6f expression in A2A-Cre mice and imaging with a miniaturized microscope (miniscope) in a freely moving mouse.
Stem Cells
In a collaboration with Dr. Leslie Thompson (UC Irvine) our lab is examining the electrophysiological properties of human neural stem cells implanted in the striatum and cortex of mice genetically altered to mimic Huntington’s Disease. In these mice transplants improve motor deficits, rescue synaptic alterations, are contacted by nerve terminals from mouse cells, and are electrophysiologically active.
Disease-modifying activity is suggested by the reduction of aberrant accumulation of mutant HTT protein and expression of brain-derived neurotrophic factor (BDNF) in these model systems. These findings hold promise for future development of stem cell-based therapies.
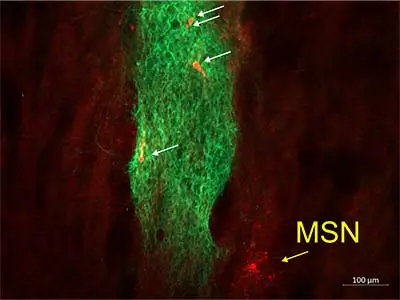
Image of human neural stem cells (hNSCs, green) immunostained with the human stem cell marker, SC121 in the striatum of an R6/2 mouse. A biocytin-filled medium-sized spiny neuron (yellow arrow) was recorded near the stem cell graft. hNSCs within the stem cell graft were also recorded and filled with biocytin (white arrows).
